From Endosymbiosis to Horizontal Gene Transfer: Has Scientific Research Uprooted Darwin’s Tree?
September 15, 2019
Famous for popularizing the once disregarded endosymbiosis theory, biologist Lynn Margulis was the ultimate iconoclast.
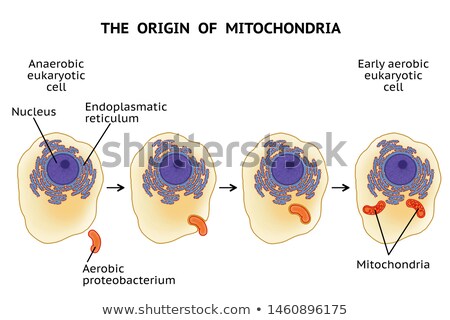
Although Dr. Margulis began her career researching the uncontroversial Eugena gracilis, she had gravitated to the intersection between genetics and evolutionary biology. As she took classes at the University of Chicago under Professor Hans Ris, she quickly pored over his research, which found evidence for the existence of DNA within chloroplasts. So-called cytoplasmic inheritance quickly challenged Mendelian inheritance; if DNA existed within a cell’s cytoplasm, not just in the chromosomes of the nucleus, that meant that female ova had an outsized influence on genetic identity compared to male sperm or pollen. The upset was not over, though. Oddly enough, Ris’s work also discovered that chloroplasts resembled cyanobacteria with their structural oddities, namely DNA fibrils and a double membrane. Could it be that chloroplasts had been bacteria?
As she left her marriage with astrobiologist Carl Sagan and forged a new identity for herself, Dr. Margulis introduced into popular culture this theory of endosymbiosis, postulating that mitochondria, chloroplasts, and undulipodia had once been bacteria that were incorporated symbiotically into eukaryotic cells. Though the third organelle was later found to not be of bacterial origin, her magnum opus, titled The Origin of Eukaryotic Cells in homage to Charles Darwin’s On the Origin of Species, prompted doubts about Darwin’s pre-molecular biology theory of evolution.
Dr. Margulis’s much criticized and praised theory ascribes today’s diversity of life not primarily towards the environment’s selection of random mutations, as Darwin had believed, but to novel moments of symbiosis. However, as much as it disrupted the status quo, it would take twenty years, the 1990s, for horizontal gene transfer to fully challenge our entire notions of evolution.
During the 1920’s, during the pre-antibiotic era, before biologists had even appreciated the role of DNA, researcher Fred Griffith noticed an anomaly. He had been studying the bacterium Streptococcus pneumoniae, and quickly discovered that each of the four types of pneumococcus existed in a virulent and mild form. By injecting the dead, albeit virulent, form of Type II pneumococcus and the alive mild form of Type I pneumococcus, he was able to kill a mouse. This should not have happened. Drawing blood, he found that the living Type I had become virulent. How?
It turns out, genes were moving across strains in a process dubbed horizontal gene transfer. And it was especially relevant. Having quickly become confounded by the improbability of antibiotic resistance given Darwin’s theory of random mutations, researchers wondered if another process existed. Indeed they found that combining Shigella resistant to streptomycin and tetracycline with nonresistant E. coli resulted in the antibiotic-resistant genes transferring to the E. coli, a daunting notion that has culminated in today’s struggles against VRSA and MRSA.
However, this phenomenon still raises the question of how horizontal gene transfer relates to the eukaryotes that comprise as broad a domain as humans and plants. To examine this question, scientists have turned to the age-old model organism, Drosophila anassae, a species of fruit fly. Across the world, Drosophila and other insects are plagued by a germ-cell parasite, the bacterium Wolbachia; notable for only being able to infect female ova, the parasite employs a variety of methods to increase the frequency of females in the population. Scientists, after infecting Drosophila with Wolbachia and subsequently employing an antibiotic, observed the presence of horizontal gene transfer across domains. Wolbachia genes had clearly been incorporated into the fly’s nuclear genome.
Since then, scientists have linked instances of horizontal gene transfer to cancerous mutations and have even discovered that the functioning of the placenta, a mammalian hallmark, is dependent on the presence of a chance endogenous retrovirus, without which fetuses quickly perish. As we learn more about our own evolutionary origins, we are thrust into an intense philosophical discussion over what it means to be truly human. Darwin’s view, though remarkable given its accuracy in an era before the advent of bioinformatics and microbiology, never accounted for the mosaicesque bacterial and viral nature of our genomes, and in turn our identities. Rather than make us less unique, the genetic history within each one of us serves to emphasize the oneness of all forms of life. Empowered by the realization that we are not situated on a pedestal above other animals, we have an obligation to adopt a less anthropocentric view of our role on Earth.
Source: The Tangled Tree: A Radical New History of Life by David Quammen









